The global clinical trial management systems market is projected to garner a valuation of around US$ 1.3 Billion in 2022.
The Clinical Trial Management Systems (CTMS) market refers to the software systems that are used to manage clinical trials, including planning, tracking, and reporting. The market for CTMS is driven by the increasing demand for new drugs and medical devices, which require extensive clinical trials to ensure safety and efficacy. The global CTMS market size was valued at USD 1.02 billion in 2020 and is expected to grow at a compound annual growth rate (CAGR) of 12.1% from 2021 to 2028. The market growth is driven by the increasing adoption of electronic data capture (EDC) systems, rising demand for improved efficiency in clinical trial operations, and the need for compliance with regulatory requirements.
The key players in the CTMS market include Oracle Corporation, Parexel International Corporation, Bioclinica, Inc., Medidata Solutions, Inc., Veeva Systems Inc., IBM Corporation, and ArisGlobal LLC, among others. These companies offer a range of CTMS software and services to pharmaceutical and biotechnology companies, contract research organizations (CROs), and academic research institutions.
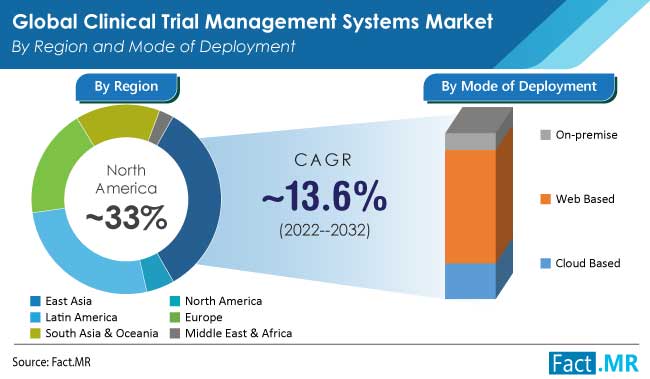
North America is the largest market for CTMS, followed by Europe and the Asia Pacific. The dominance of these regions can be attributed to the presence of a large number of pharmaceutical and biotechnology companies, academic research institutions, and CROs, as well as the well-established regulatory framework for clinical trials.
Overall, the CTMS market is expected to grow in the coming years, driven by the increasing demand for new drugs and medical devices, the adoption of EDC systems, and the need for more efficient and compliant clinical trial operations
Download Free Sample Copy of this Report – https://www.factmr.com/connectus/sample?flag=S&rep_id=832
Key Segments Covered in Clinical Trial Management System Industry Survey
Competitive Landscape
How is Growing Adoption of Web-based Mode of Deployment Impacting Clinical Trial Management System Sales?
The last five years have seen a notable rise in demand for web-based clinical trial management systems. Real-time information access and rapid results analysis are likely to increase demand for the same. Additionally, the virtual storage option has shown to be cost-effective for the biotechnology and pharmaceutical industries.
In 2020, the revenue share for web-based clinical trial management systems was about 68%. Although there are still issues, web-based clinical trial management is the end users’ preferred deployment model due to its adaptability, low cost, and cutting-edge technology. As a result, by the end of the forecast period, the web-based mode of deployment will be valued at US$3.2 million. During the assessment period as well, web-based clinical trial management systems are likely to remain popular. End users now have the resources to centralize computational and research tools thanks to the move to web-based clinical trial management.
However, due to problems with data security and privacy, the clinical trial industry encounters resistance. There may be less uncertainty if there are cloud-based services and platforms that can optimize the cost of clinical trial management systems through the use of big data technology.
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions.
Jun 23, 2023